Purpose: The Valence Shell Electron-Pair Repulsion Theory, or the VSEPR theory, is a method used in order to predict the shape of a molecule due to its chemical formula. The experiment designed here is intended to allow for an understanding of molecular shapes and the arrangement of atoms in a molecule.
VSEPR Theory | CK-12 Foundation
1: Review of Chemical Bonding Expand/collapse global location 1.3: The Shapes of Molecules (VSEPR Theory) and Orbital Hybridization

Source Image: study.com
Download Image
a. The VSEPR (Valence Shell Electron Pair Repulsion) is a theory that predicts the molecular shape, or structure, of a compound with the least electron repulsions. This theory is based on the central atoms’ electron groups and lone pairs. A. Can the structure of simple molecular substances be illustrated by drawing or building models?

Source Image: coursehero.com
Download Image
SOLVED: Molecular Models/VSEPR Theory/Polarity Datasheet Electron Pair Molecular Molecule Lewis Dot Structure Arrangement Geometry Polarity H2S H-S- CH3Cl H2O SiO2 Cl2 Boron Trifluoride C2H6 H-C=C-H SO2 NF3 CH2O H-C-H CCl4 CH3CHCH2 H-C=C-H
Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope, most of our information about molecular structure comes from often this information enables us to piece together a 3-dimensional picture of the molecule.

Source Image: coursehero.com
Download Image
Lab Report For Vsepr Theory And Shapes Of Molecules
Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope, most of our information about molecular structure comes from often this information enables us to piece together a 3-dimensional picture of the molecule.
Science Chemistry Chemistry questions and answers Lab Report for VSEPR Theory and Shapes of Molecules Fill the following tables. Do not indicate polarity for charged species (ions). HCN 1. Lewis Structure 2. Perspective drawing 3. Number of atoms bonded to central atom 5. Electronic geometry: 4.
Lab 10 2.jpg – EXPERIMENT 17: Lewis Dot Structure / VSEPR Theory REPORT FORM Name Antonio Lopez Instructor Nawal Sharma Date April 11 2020 Partner’s | Course Hero
Observations and Experimental Part 1: Basic Shapes Step 1: Apply the VSEPR model to predict the shapes of the following molecules and measure the bond angles. Comment on how closely the observed bond angles agree with the ideal values. Molecule Predicted Shape Bond Angle (o) Expected angle PF6 – Octahedral 89° 90° (very close)
SOLVED: Data Sheet: Experiment Laboratory Manual – Chemical Bonding and Molecular Shapes with VSEPR Theory Chemical Formula Skeleton of the model Including bond angles and molecular shape. Use multiple lines to represent
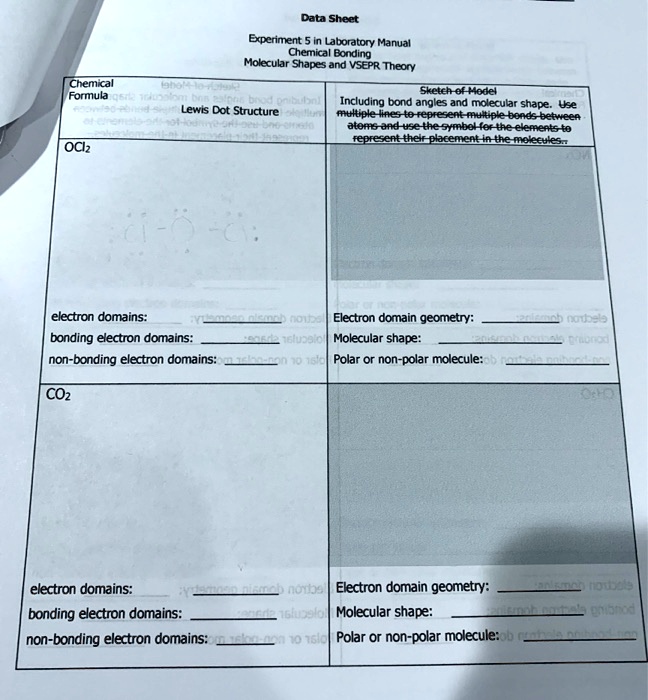
Source Image: numerade.com
Download Image
Lab Report 6 – Summary: In this experiment we will be using the WebCSD database to study shapes of – Studocu
Observations and Experimental Part 1: Basic Shapes Step 1: Apply the VSEPR model to predict the shapes of the following molecules and measure the bond angles. Comment on how closely the observed bond angles agree with the ideal values. Molecule Predicted Shape Bond Angle (o) Expected angle PF6 – Octahedral 89° 90° (very close)

Source Image: studocu.com
Download Image
VSEPR Theory | CK-12 Foundation
Purpose: The Valence Shell Electron-Pair Repulsion Theory, or the VSEPR theory, is a method used in order to predict the shape of a molecule due to its chemical formula. The experiment designed here is intended to allow for an understanding of molecular shapes and the arrangement of atoms in a molecule.

Source Image: flexbooks.ck12.org
Download Image
SOLVED: Molecular Models/VSEPR Theory/Polarity Datasheet Electron Pair Molecular Molecule Lewis Dot Structure Arrangement Geometry Polarity H2S H-S- CH3Cl H2O SiO2 Cl2 Boron Trifluoride C2H6 H-C=C-H SO2 NF3 CH2O H-C-H CCl4 CH3CHCH2 H-C=C-H
a. The VSEPR (Valence Shell Electron Pair Repulsion) is a theory that predicts the molecular shape, or structure, of a compound with the least electron repulsions. This theory is based on the central atoms’ electron groups and lone pairs. A. Can the structure of simple molecular substances be illustrated by drawing or building models?
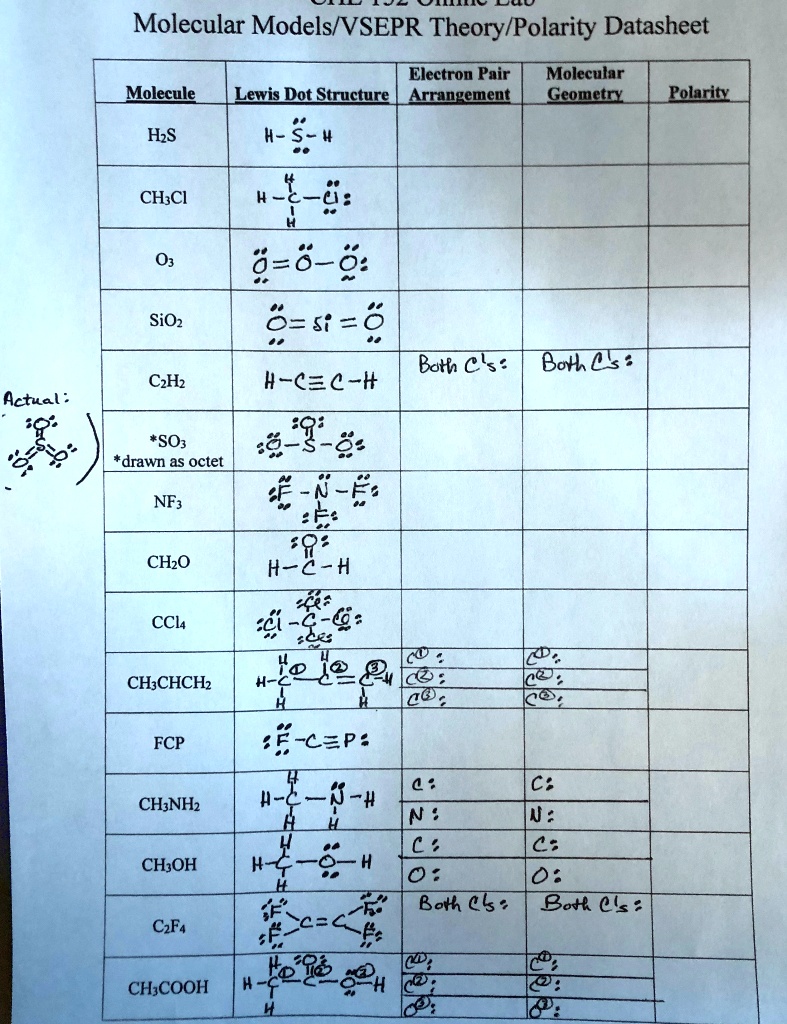
Source Image: numerade.com
Download Image
PPT – VSEPR THEORY: PowerPoint Presentation, free download – ID:3261093
The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. A combination of VSEPR and a bonding model, such as Lewis electron structures, is necessary to understand the presence of multiple bonds.
Source Image: slideserve.com
Download Image
SOLVED: Experiment #7 Lab Report Name: Procedure: Use your molecular model kit to make the following models. Have your model checked by your instructor. Use the model to fill in the table.
Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope, most of our information about molecular structure comes from often this information enables us to piece together a 3-dimensional picture of the molecule.
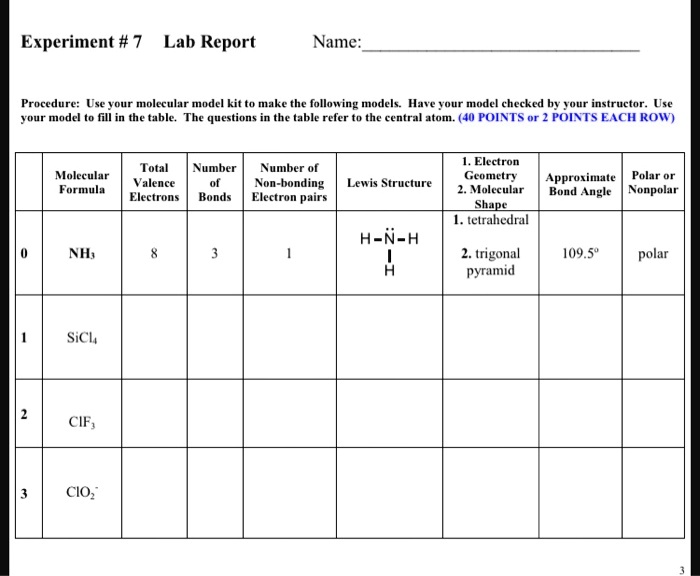
Source Image: numerade.com
Download Image
SOLUTION: VSEPR Theory notes, BSc class – Studypool
Science Chemistry Chemistry questions and answers Lab Report for VSEPR Theory and Shapes of Molecules Fill the following tables. Do not indicate polarity for charged species (ions). HCN 1. Lewis Structure 2. Perspective drawing 3. Number of atoms bonded to central atom 5. Electronic geometry: 4.

Source Image: studypool.com
Download Image
Lab Report 6 – Summary: In this experiment we will be using the WebCSD database to study shapes of – Studocu
SOLUTION: VSEPR Theory notes, BSc class – Studypool
1: Review of Chemical Bonding Expand/collapse global location 1.3: The Shapes of Molecules (VSEPR Theory) and Orbital Hybridization
SOLVED: Molecular Models/VSEPR Theory/Polarity Datasheet Electron Pair Molecular Molecule Lewis Dot Structure Arrangement Geometry Polarity H2S H-S- CH3Cl H2O SiO2 Cl2 Boron Trifluoride C2H6 H-C=C-H SO2 NF3 CH2O H-C-H CCl4 CH3CHCH2 H-C=C-H SOLVED: Experiment #7 Lab Report Name: Procedure: Use your molecular model kit to make the following models. Have your model checked by your instructor. Use the model to fill in the table.
The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. A combination of VSEPR and a bonding model, such as Lewis electron structures, is necessary to understand the presence of multiple bonds.









